Cushing’s syndrome is a collection of symptoms and signs caused by prolonged exposure to elevated levels of either endogenous or exogenous glucocorticoids. The incidence of Cushing’s syndrome is approximately 10-15 per million, and the incidence is higher in people with a history of obesity, hypertension and diabetes mellitus.
Aetiology
The most common cause of Cushing’s syndrome is the iatrogenic administration of corticosteroids. The second most common cause of Cushing’s syndrome is Cushing’s disease. Cushing’s disease should be distinguished from Cushing’s syndrome and refers to one specific cause of the syndrome, an adenoma of the pituitary gland that secretes large amounts of ACTH and in turn elevates cortisol levels.
The endogenous causes of Cushing’s syndrome include:
- Pituitary adenoma (Cushing’s disease)
- Ectopic corticotropin syndrome, e.g. small cell carcinoma of the lung
- Adrenal adenoma
- Adrenal carcinoma
- Adrenal hyperplasia
Clinical features
The clinical features of Cushing’s syndrome are highly variable and depend upon the degree of cortisol overproduction. In cases where there is only mild elevation of cortisol levels presentation can be subtle and the diagnosis difficult to make. Conversely, in long-standing cases with grossly elevated cortisol levels, the presentation can be florid and the diagnosis straightforward.
The clinical features of Cushing’s syndrome include:
- Truncal obesity and weight gain
- Supraclavicular fat pads
- Buffalo hump
- Facial fullness and plethora (‘moon facies’)
- Proximal muscle weakness and wasting
- Diabetes mellitus or impaired glucose tolerance
- Hypertension
- Skin atrophy and easy bruising
- Hirsutism
- Acne
- Osteoporosis
- Depression
- Amenorrhoea or oligomenorrhoea
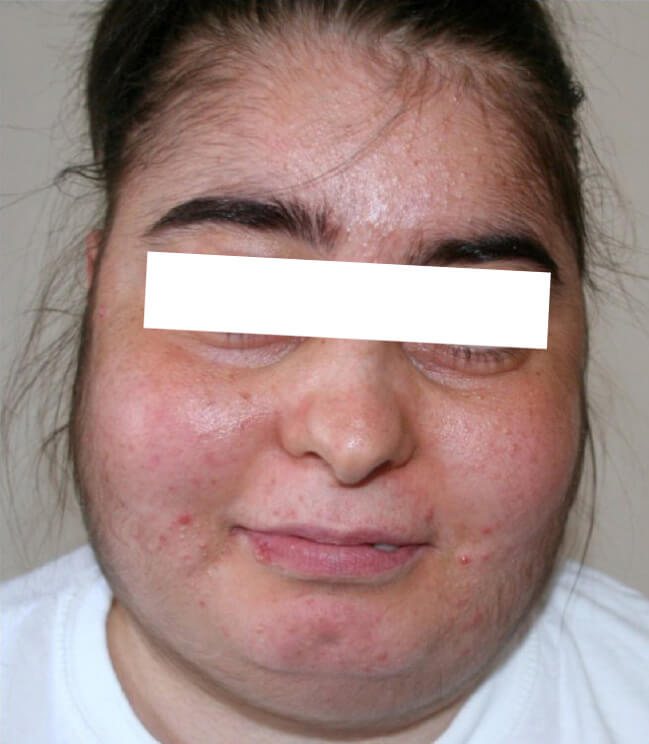
Cushingoid facies in a patient with iatrogenic Cushing’s syndrome, image sourced from Wikipedia
Courtesy of the Multidisciplinary Respiratory Medicine Journal CC BY-SA 2.5
Investigations to confirm the presence of Cushing’s syndrome
Cortisol levels vary throughout the day and are usually highest at 0900 hrs and lowest during sleep at 2400 hrs. In Cushing’s syndrome, there is a loss of the diurnal variation of the cortisol levels and levels are higher throughout the entire 24-hour period. Levels may be within the normal range in the morning but high at midnight when they are usually suppressed. For this reason, random cortisol is not a useful screening tool and is not recommended.
The main two first-line screening tests that are used are:
- 24-hour urinary free cortisol collection
- Overnight low-dose dexamethasone suppression test
24-hour urinary free cortisol collection
- A minimum of two collections should be taken and ideally three
- A diagnosis of Cushing’s syndrome can be made if >2 collections measure cortisol excretion greater than three times the upper limit of normal
- False positives can occur with physical stress (e.g. heavy exercise, trauma), mental stress (e.g. depression), alcohol or drug abuse, complicated diabetes and pregnancy
- False negatives can occur with renal failure, incomplete collection and cyclical Cushing’s syndrome.
Overnight low-dose dexamethasone suppression test (LDDST):
- 1 mg of dexamethasone is administered at 11 pm, and serum cortisol levels are measured at 8 am the following morning
- Failure to suppress cortisol to less than 50 nmol/L is suggestive of a diagnosis of Cushing’s syndrome
- Mild Cushing’s syndrome can be challenging to distinguish from normal cortisol secretion
- False positives can occur with depression, severe systemic illness, renal failure, chronic alcohol abuse, old age and with the use of hepatic enzyme-inducing drugs.
- False negatives are very rare in patients with Cushing’s syndrome.
A typical biochemical picture can also be useful to provide supporting evidence of a diagnosis of Cushing’s syndrome. The main features are:
- Hypokalaemia
- Metabolic acidosis
Investigations to identify the underlying cause of Cushing’s syndrome
The aim of the second-line tests is to identify the underlying cause of Cushing’s syndrome. It can sometimes be difficult to pinpoint the cause, and a process of elimination is generally required.
Plasma ACTH levels:
- Secretion of ACTH is pulsatile and shows a diurnal variation, with levels highest at 8 am and lowest at midnight
- Isolated random ACTH levels are not useful, and all results should be interpreted in combination with simultaneously taken cortisol levels
- ACTH levels are typically suppressed in adrenal adenoma and adrenal carcinoma
- ACTH levels are typically elevated in ectopic production and, to a lesser degree, in pituitary driven disease
High-dose dexamethasone suppression test (HDDST):
- The HDDST is useful when baseline ACTH levels are equivocal
- 2 mg if dexamethasone is administered 6-hourly for 48 hours are measured at 8 am the following morning
- A >90% reduction in basal urinary free cortisol levels is supportive of a diagnosis of a pituitary adenoma
- Ectopic ACTH can also cause suppression, but to a lesser degree.
Inferior petrosal sinus sampling (IPS):
- IPS is useful when patients have confirmed ACTH-dependent Cushing’s syndrome
- IPS is an invasive procedure in which ACTH levels are sampled from the veins that drain the pituitary gland and compared with serum ACTH levels in the peripheral blood
- It is performed in conjunction with corticotrophin-releasing hormone (CRH) stimulation
- A baseline and stimulated ratio of IPS to peripheral ACTH of greater than 2 is consistent with a pituitary adenoma.
- A baseline and stimulated ratio of IPS to peripheral ACTH of less than 1.8 is suggestive of ectopic ACTH
Other useful investigations include:
- Pituitary CT or MRI scan – to identify pituitary adenoma
- Chest X-ray – to identify small cell carcinomas of the bronchus causing ectopic ACTH production
- Chest and abdominal CT scan – to identify adrenal tumours, carcinoid tumours or small cell carcinomas of the bronchus if ectopic ACTH production is suspected
- Plasma CRH levels – to identify ectopic CRH production
Management
The management of Cushing’s syndrome is dependent upon the underlying cause.
Iatrogenic Cushing’s syndrome secondary to corticosteroid administration:
- Treated by lowering or withdrawing steroids if possible.
Pituitary adenoma (Cushing’s disease):
- Surgery is the treatment of choice, usually by a trans-sphenoidal approach
- The goal is to render serum cortisol undetectable
- Post-operative radiotherapy is sometimes also required to achieve this
- Bilateral adrenalectomy is less commonly performed but is occasionally needed
- Remission is achieved in 75-80% of cases
Adrenal tumours:
- Unilateral adrenalectomy with excision of the adenoma or carcinoma is the treatment of choice
- Metyrapone should be administered before surgery to achieve clinical remission (this is associated with reduced perioperative morbidity and mortality).
Carcinoid tumours:
- Surgical excision is the treatment of choice if possible
- If the tumour is inaccessible to surgery radiotherapy and/or long-term chemotherapy is indicated.
Header image used on licence from Shutterstock
Thank you to the joint editorial team of www.plabprep.co.uk for this ‘Exam Tips’ post.